Getting overwhelmed by the news is an everyday struggle for those wishing to stay in touch with the latest news. Our newsletter should make it easier for those interested in pharmacovigilance regulations, advancement in the relevant use of artificial intelligence, and personal growth. Approaching with care, the same as we provide PV services, we thoughtfully pick articles every month to keep you updated.
Guideline on good pharmacovigilance practices (GVP) - Module VI Addendum II – Masking of personal data in individual case safety reports submitted to EudraVigilance.
To date, signals of adverse reactions to herbal medicines have not been systematically reviewed, limiting pharmacovigilance of herbal medicines because of a lack of data.
We’ve looked at woke from all sides now. Woke AI, that is. It’s become a widely discussed thing, thanks to President Donald Trump’s July 23 executive order “Preventing Woke AI in the Federal Government.
And much more...

Questions and answers on Implementing Regulation (EU) 2025/1466: Amendment of Regulation (EU) No 520/2012 and Conclusion of the Signal Detection in EudraVigilance Pilot by marketing authorisation holders

IRIS guide for applicants - How to create, submit and manage IRIS applications, for industry and individual applicants

Guideline on good pharmacovigilance practices (GVP) - Module VI Addendum II – Masking of personal data in individual case safety reports submitted to EudraVigilance

We’ve looked at woke from all sides now. Woke AI, that is. It’s become a widely discussed thing, thanks to President Donald Trump’s July 23 executive order “Preventing Woke AI in the Federal Government.”
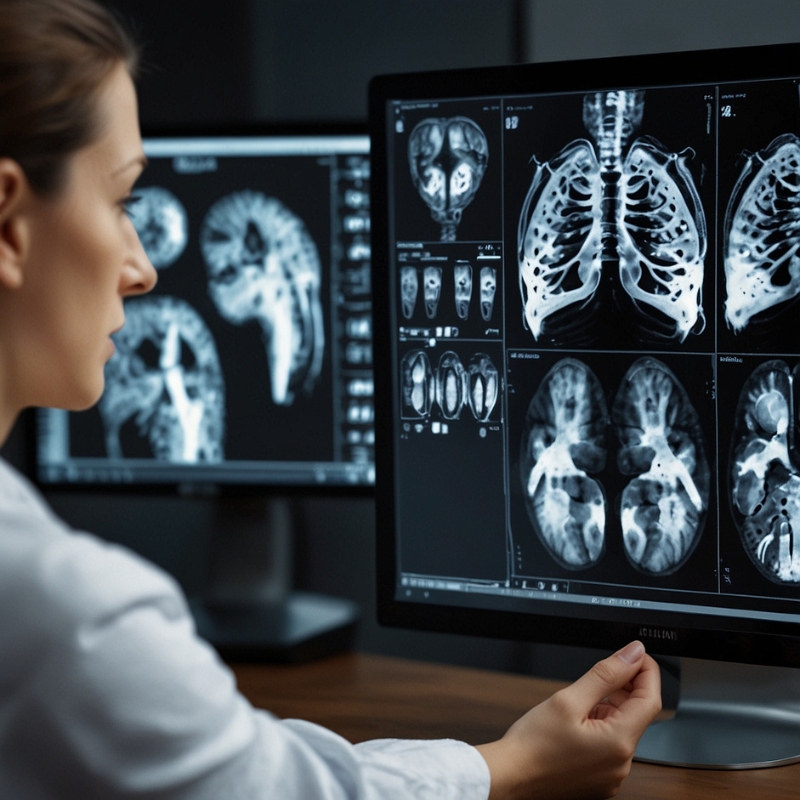
Medical radiology reports play a crucial role in diagnosing various diseases, yet generating them manually is time-consuming and burdens clinical workflows.
Within healthcare, artificial intelligence and quality improvement have some things in common.

To date, signals of adverse reactions to herbal medicines have not been systematically reviewed, limiting pharmacovigilance of herbal medicines because of a lack of data.

Affective disorders, particularly depression, are common among women of childbearing age, and pregnancy often exacerbates symptoms. Antidepressants are often required for treatment, but adherence during pregnancy is variable. Although some studies suggest potential risks to the foetus, many cannot rule out confounding by indication.

Imbalanced datasets have been a persistent challenge in bioinformatics, particularly in the context of drug-drug interaction (DDI) risk level datasets. Such imbalance can lead to biased models that perform poorly on underrepresented classes.
Pictures used in this newsletter were generated by AI.